Tyvek® Offers Unmatched Sterilization Compatibility
Article | February 19, 2018

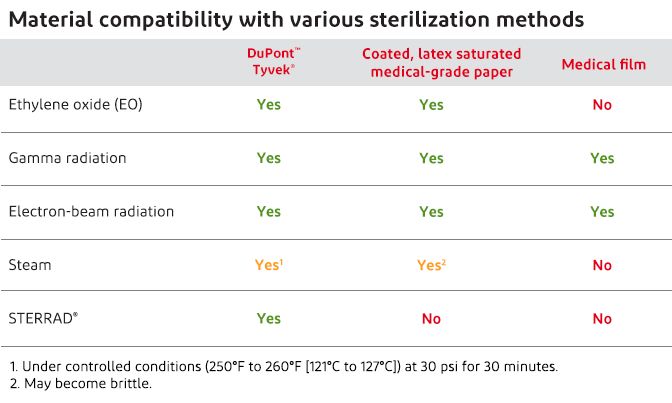
Unlike medical-grade papers and films, DuPont™ Tyvek® offers sterilization compatibility with all of the most commonly used methods for sterilizing medical devices. These include: ethylene oxide (EO), gamma, electron-beam, hydrogen peroxide gas plasma and steam (under controlled conditions). That’s because Tyvek® is made of 100% high-density polyethylene (HDPE), which is extremely stable when exposed to sterilant gases and high-energy sterilization processes. What’s more, Tyvek® is specially engineered to enable sterilant gases and steam to penetrate and escape quickly.
When it comes to sterilization compatibility, Tyvek® offers the broadest range of options. And no matter which process you choose—EO, gamma, electron-beam, steam (under controlled conditions) or low-temperature oxidative sterilization processes—Tyvek® will retain its superior protective properties of microbial barrier and strength, as well as its color and flexibility.
Ethylene Oxide [EO]
Ethylene oxide (EO) does not readily adsorb on Tyvek® and is released more rapidly than from cellulosic materials such as medical-grade papers, including synthetic fiber-reinforced paper. Tyvek® maintains its superior strength and microbial barrier properties after EO sterilization.
Gamma Radiation
After exposure to gamma radiation up to 100 kGy, Tyvek® maintains its superior microbial barrier and the impact on strength properties is limited. These properties are also maintained after irradiation followed by exposure to accelerated and real-time aging.
Electron-Beam Radiation
After exposure to electron-beam radiation up to 100 kGy, Tyvek® maintains its superior microbial barrier and strength properties. Although specific studies of electron-beam sterilization followed by aging have not been conducted, we have not seen any effects other than those shown after gamma radiation and aging. This is because HDPE is radiation stable.
Hydrogen Peroxide Gas Plasma
Tyvek® 4057B is suitable for use with the STERRAD® Sterilization System from Advanced Sterilization Products (ASP), Division of Ethicon Inc., a Johnson & Johnson company. This sterilization method uses low-temperature gas plasma to enable sterilization of heat-labile devices.
Caution should be used when choosing methodologies for package integrity testing after multiple doses of low-temperature oxidative sterilization because the water resistance of the material can be altered.
Medical-grade papers, including autoclave paper pouches, are not acceptable for use with the STERRAD® System because cellulosic materials neutralize the sterilizing agent.
ASP has developed a complete range of sterilization pouches and rolls using Tyvek® 4057B for the STERRAD® System.
More information about the STERRAD® System, including cycle times and performance details, can be found on its website.
Other Low-Temperature Oxidative Methods
In addition to hydrogen peroxide gas plasma sterilization, other low-temperature oxidative sterilization methods such as vaporized hydrogen peroxide, nitrogen dioxide and low-temperature peracetic acid vapor gas plasma sterilization have been introduced. DuPont does not have test data for the performance of Tyvek® in each of these low-temperature oxidative sterilization processes; however, the manufacturers of these sterilizers usually have data on Tyvek® compatibility. Please contact the sterilizer manufacturer for more information.
Steam
Tyvek® has been shown to meet packaging criteria for steam sterilization under controlled conditions (250°F to 260°F [121°C to 127°C] at 30 psi for 30 minutes). Tyvek® continues to be superior to medical-grade paper when strong, low-linting packaging is required. Tyvek® retains its dimensional stability and integrity with no discoloration when steam sterilized under the controlled conditions mentioned above. Rigid or semi-rigid trays restrict potential shrinkage and wrinkling, which can result in a smoother/tighter lid. Shrinkage of Tyvek® after steam sterilization is less than 1.6%. Tensile strength, microbial barrier and Gurley Hill porosity of Tyvek® are maintained after steam sterilization under the controlled conditions stated above.
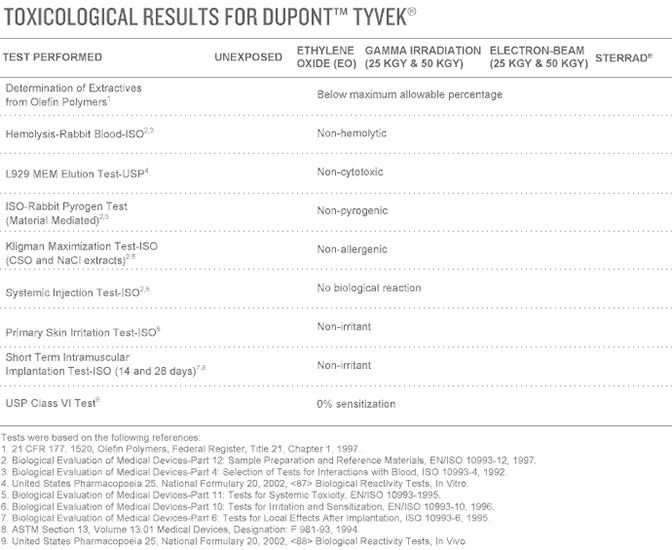
Biocompatibility
Biological evaluation of Tyvek® styles for medical and pharmaceutical packaging was performed using testing methodologies according to ISO 10993 and United States Pharmacopeia (USP). In all cases, the styles met all the acceptable performance criteria. This also held true for samples of Tyvek® tested after exposure to sterilization by the EO, gamma and electron-beam processes, proving that Tyvek® meets all the acceptable performance criteria after sterilization. The results of the testing indicate biocompatibility—even after sterilization.